Today i'm going to share some very very basic concepts/information on solar energy-specially on Solar Photo Voltaic. the reason behind this post is for some viewers of my blog asking me(actually mailing me) what is solar PV, how it works etc. So let's start discussing!!
The sunlight reaching the Earth’s surface generates six thousand times more than the estimated 15 Terawatts (TW) of power consumed around the globe every year. It’s no wonder then, that mankind has long sought to capture and use the power of the sun. The challenge has always been the efficiency of capture and distribution.The current generated by the flow of electrons, when metal contacts are placed above and below the photovoltaic cell, can be extracted for external use. Solar cells, then, are the essential component in photovoltaic systems.
 To generate sufficient voltage, multiple cells are then interconnected to make up a solar panel.By leveraging capabilities of the photovoltaic process, as photons of sunlight, comprised of energy particles of different wavelengths hit a photovoltaic cell, their energy is transferred to the electrons which become part of a current in an electrical circuit.
To generate sufficient voltage, multiple cells are then interconnected to make up a solar panel.By leveraging capabilities of the photovoltaic process, as photons of sunlight, comprised of energy particles of different wavelengths hit a photovoltaic cell, their energy is transferred to the electrons which become part of a current in an electrical circuit.How Silicon Makes a Solar Cell
Silicon has some special chemical properties, especially in its crystalline form. An atom of silicon has 14 electrons, arranged in three different shells. The first two shells -- which hold two and eight electrons respectively -- are completely full. The outer shell, however, is only half full with just four electrons. A silicon atom will always look for ways to fill up its last shell, and to do this, it will share electrons with four nearby atoms. It's like each atom holds hands with its neighbors, except that in this case, each atom has four hands joined to four neighbors. That's what forms thecrystalline structure, and that structure turns out to be important to this type of PV cell.
The only problem is that pure crystalline silicon is a poor conductor of electricity because none of its electrons are free to move about, unlike the electrons in more optimum conductors like copper. To address this issue, the silicon in a solar cell has impurities -- other atoms purposefully mixed in with the silicon atoms -- which changes the way things work a bit. We usually think of impurities as something undesirable, but in this case, our cell wouldn't work without them. Consider silicon with an atom of phosphorous here and there, maybe one for every million silicon atoms. Phosphorous has five electrons in its outer shell, not four. It still bonds with its silicon neighbor atoms, but in a sense, the phosphorous has one electron that doesn't have anyone to hold hands with. It doesn't form part of a bond, but there is a positive proton in the phosphorous nucleus holding it in place.
When energy is added to pure silicon, in the form of heat for example, it can cause a few electrons to break free of their bonds and leave their atoms. A hole is left behind in each case. These electrons, called free carriers, then wander randomly around the crystalline lattice looking for another hole to fall into and carrying an electrical current. However, there are so few of them in pure silicon, that they aren't very useful.
But our impure silicon with phosphorous atoms mixed in is a different story. It takes a lot less energy to knock loose one of our "extra" phosphorous electrons because they aren't tied up in a bond with any neighboring atoms. As a result, most of these electrons do break free, and we have a lot more free carriers than we would have in pure silicon. The process of adding impurities on purpose is called doping, and when doped with phosphorous, the resulting silicon is called N-type ("n" for negative) because of the prevalence of free electrons. N-type doped silicon is a much better conductor than pure silicon.
 The other part of a typical solar cell is doped with the element boron, which has only three electrons in its outer shell instead of four, to become P-type silicon. Instead of having free electrons, P-type ("p" for positive) has free openings and carries the opposite (positive) charge.
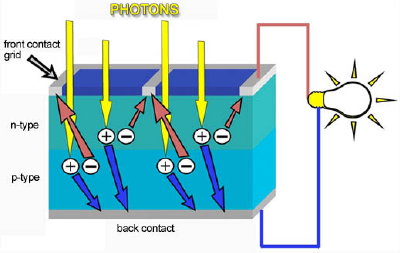
The other part of a typical solar cell is doped with the element boron, which has only three electrons in its outer shell instead of four, to become P-type silicon. Instead of having free electrons, P-type ("p" for positive) has free openings and carries the opposite (positive) charge.There are a few different technologies that support the photovoltaic process. The most commonly deployed solar panels in today’s market are comprised of solar cells that are made of silicon. Silicon (sand) is the second most abundant element in the Earth’s crust and the very same element that makes computer chips possible. The cell consists of two or more thin layers of semiconductor material. The layers are given opposite charges – one positive, one negative. When sunlight strikes the solar cell, electrons are knocked loose and move toward the treated front surface of the solar cell. This creates an electron imbalance between the front and back of the cell and causes electricity to flow – the greater the intensity of light, the greater the flow of electricity.
With steadily rising costs for carbon based fuels, concerns over their impact on the environment, and various incentives in place to help solar adoption, continued innovation in photovoltaics continues to helping to deliver on the power of the sun.
Thank you for reading!!














QUANTUM BINARY SIGNALS
ReplyDeleteGet professional trading signals delivered to your cell phone daily.
Start following our trades today and gain up to 270% a day.